Cell plating and exposure
This video shows how to plate cells and expose them to compounds.
- Tineke Bijl
-
(0)
- 0 enrolled students

Description
Non-interactive video download link (mp4).
The script that has been used to create the video shown above can be downloaded as excel script file.
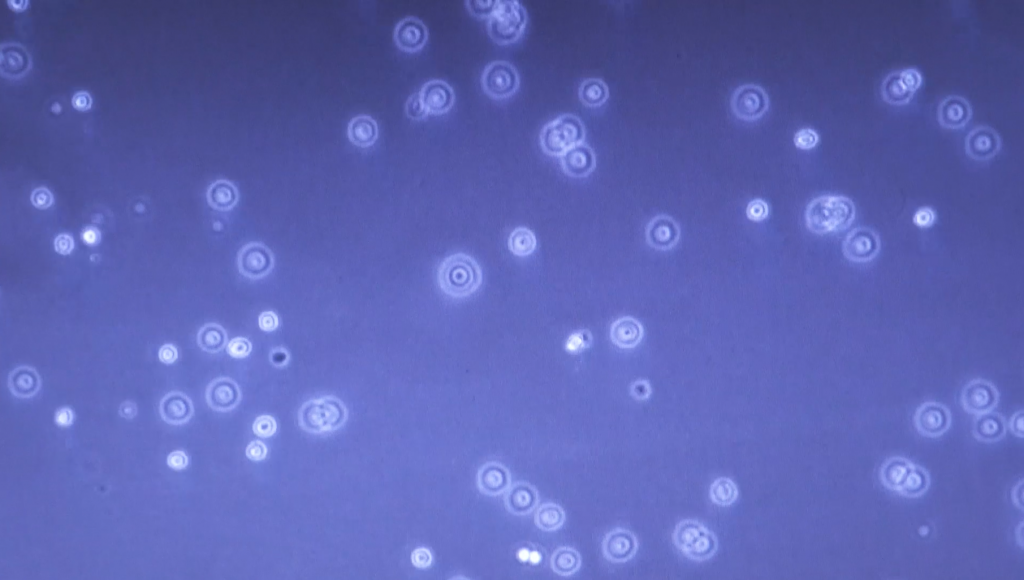
| 1 | Each team will receive two T25 cellculture flasks: one flask with TNCB1 and one with TNBC2. Take the flasks from the CO2 incubator and inspect the cells under the light microscope:
|
| 2 | Sterilize the suction needle and remove the medium from the flask carefully without touching the cells. |
| 3 | Pipet 2 mL of sterile PBS into the flask and slowly rinse the cells by swirling. |
| 4 | Sterilize the suction needle and remove the PBS from the cell culture flask. |
| 5 | Pipet 1 mL of 0.25% Trypsin and swirl solution over the culture surface. Put your cells back in the incubator for 1-2 minutes (TNBC2) or at least 5 minutes (TNBC1) |
| 6 | After incubation, inspect the cells to see if cells have detached (tapping may help to detach cells further), otherwise place the flask in the incubator for another 1-3 minutes. |
| 7 |
|
| 8 |
|
| 9 |
|
| 10 | Based on your cell count per mL, calculate the volume of cells you need to dilute in order to seed out 1500 cells/well (TNBC1) and 1000 cells/well (TNBC2) in a final volume of 100 µL/well. NOTE: Prepare a cell suspension enough for 40 wells per plate for each celline although you only need 24 per plate for each celline! This is to make sure that you always have enough cell suspension. |
| 11 |
|
| 12 | Incubate the cells overnight at 37°C and 5% CO2. |
| 13 |
|
| 14 |
|
After seeding the cells, the 96-wells plates will be placed in the incubator, why?
Feedback if correct: The seeding process is very stressful for cells. So, it is better to let the cells rest before continuing with the experiment. Also, the cells need some time to attach to the bottom of the plate, the cells are floating in the medium after seeding. Feedback if b: This is a pleasant incidental, but not the main reason why the cells are placed in the incubator Feedback if d: This is partly true but not the main reason why the cells should be placed in the incubator. |
Before seeding your cells, it is necessary to resuspend your cell suspension. Why is this resuspension required?
|
Enter the cell density and calculate how much cell suspension you need to prepare sufficient diluted solution for 1500 TNBC1 cells in 100 µL per well (use TNBC1 as example).
_d_ µL cell suspension required to obtain the diluted solution. Feedback if b is not 4: How much volume should each well contain? So what is the total for all 40 wells? Feedback if d is not 15000*4/(c*10^6): You did not correctly calculate how much cell suspension you need to take for a dilution with a total volume of 4 mL. Feedback if correct: Given your concentration of [mathresult|c] ×106 cells/mL you should prepare a dilution by taking [mathresult|d] µL and filling up with medium to a total of 4 mL. |
Why is it necessary to add PBS to your flask after removal of the culture medium?
Feedback if correct. PBS is needed to wash the cells and get rid of medium components like serum, which inactivates trypsin. Without a PBS washing step will not/hardly detach from your flask. Feedback if a: PBS is not needed to remove dead cells, you can simply discard to medium to do so. Feedback if incorrect: No try again. One option is correct. |
Download zip to import in LabBuddy.
Please make sure that your product exists and valid for this course
- Skill levelIntroduction video
- CategoryCell biology
Related videos
-
Free
Cell counting
Copyright information
This video is created by Leiden Academic Centre for Drug Research (LACDR), Faculty of Science at Leiden University under a open Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. When using this video in its original version please refer to www.labprep.video. When adapting the video, mention the source ‘adapted based on the original version that is created by the labprep.video team’. It is not allowed to use the video for commercial purposes without consultation with the creators. You can contact us via info@labprep.video.